The Basics of pH Measurement
pH is the measurement of hydrogen ion concentration, all substances of an aqueous nature have the ability to be measured for it’s pH measurement. These values typically ranges from 1 to 14, 7 being the neutral value in the field. This typically means that where the acidity of a solution increases as the number decreases meaning 1 is acidic, this in turn means 14 is an alkaline solution. The units used to measure the hydrogen content this is known as moles per litre. p[H] was the original symbol used to illustrate pH, but later replaced in favour of the more relatable pH.
The Litmus Method
There are various methods of measuring pH, one such way most people would likely be familiar with is the pH test paper, commonly used in schools for science classes, this is paper (the indicator) dipped into the test liquid, this then reveals the nature of the liquid by changing colour to match the liquids pH levels.
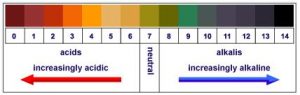
The standard for most tests in this case would be dependant upon the paper used. A good example here would be the standard litmus paper, one version of the paper could be the paper turns red upon being dipped into an acidic solution, or the reverse, the sheet turns blue when dipped into an alkaline solution. Although a good starting point into the world of pH, it can give misleading results, due to having a low degree of accuracy.
Electronic pH Measurement Devices
These are a much more scientific and reliable method of getting a true pH measurement of aqueous solutions. These normally combine some form of electronic components used in a potentiometric meter such as electrodes. The measurement is taken and then displayed as such on a digital display. Calibration of the metre is normally a standard before testing on actual solutions you are testing, known as buffer solutions that cover the spectrum of pH values to get a more accurate reading. The types of variations of metres available are wide and readily available, from pen like devices to full on laboratory equipment.
